CESR Applications for Clinical and Radiation Oncologists
- December 09, 2022
This article delves into the specific guidance on GMC applications for entry onto the UK Specialist Register through the Certificate of Eligibility for Specialist Registration (CESR) for clinical oncologists.
We’ll cover the eligibility criteria, application process, and most importantly the required evidence, along with some other topics, summarised in the headings below:
- What is CESR and who is it for?
- Do overseas oncologists need FRCR (Oncology) for CESR?
- What is the CESR equivalence process?
- What evidence is required for a CESR in clinical oncology?
- Where will I find this evidence?
- How do I submit a CESR application?
- How long does it take to complete?
- How much does CESR cost?
- How long does it take to receive a decision?
- Do I have to complete CESR before I can work in the UK?
- #IMG Tips
Skip ahead to the relevant section if you know what you’re looking for.
An Introduction to CESR
CESR, or the Certificate of Eligibility for Specialist Registration, is the route to specialist registration for doctors who have not completed a GMC-approved training programme, but can demonstrate that their specialist training, qualifications and experience are equivalent to the requirements for the award of the CCT in the UK.
CESR holders can be appointed to substantive (or permanent) consultant positions in the NHS. As a clinical or radiation oncologist, attaining specialist registration will mean you are qualified to practice independently as a consultant in the NHS.
Oncologists must prove to the GMC that their specialist training or specialist qualifications, when considered together, are equivalent to a CCT in the specialty in question.
In order to be eligible for CESR, doctors should have undertaken a minimum of 6 months training or obtained a specialist qualification and acquired specialist experience or knowledge as a clinical oncologist within a non-training post.
Overseas doctors do not require CESR before moving to the UK to work in the NHS. Often, experienced oncologists will secure a post in the UK, and work towards CESR whilst in post.
FRCR (Oncology) for Specialist Registration
Whilst it is always beneficial to complete FRCR (Oncology), particularly for doctors who have trained outside the UK or an EEA country, overseas doctors looking to join the Specialist Register do not need to have completed the Royal College postgraduate exams.
The standard test of knowledge in the Clinical Oncology curriculum is the FRCR exams, so passing these exams confirms the attainment of the competencies of the core curriculum.
FRCR (Oncology) is only a requirement for doctors looking to attain Specialist Registration via the CCT route.
Similarly, UK trainees would have completed MRCP (UK) before beginning their clinical oncology training, which is also not required for oncologists going through the CESR route.
However, CESR applicants who have not successfully completed these exams must provide alternative evidence that demonstrates equivalent knowledge to oncologists who have passed the FRCR (Oncology) exams and the appropriate level of non-oncology expertise and that you can appropriately manage the acutely unwell patient.
Even if the competencies covered by the exam require something that someone in your position would not routinely undertake (in your sub-specialty for example), you must still provide evidence of it – as the evaluators will not make assumptions outside the evidence presented.
You can read more about the evidence required in the specialty specific guidance here.
The CESR Equivalence Process
Equivalence refers to the process of assessing an overseas applicant’s training and experience against the current clinical oncology training programme requirements, in order to be awarded CESR.
The equivalence process involves submitting a written body of evidence to the GMC, consisting of:
- training and/or competence; AND
- skills and knowledge
The Royal College of Radiologists will assess each application against the relevant curriculum before providing a recommendation to the GMC, who will then make a decision.
Please note that Equivalence procedures are the responsibility of the GMC. Applications are made through their Certification Department and initial enquiries should be directed there.
Evidence Requirements for CESR in Clinical Oncology
Skills & Experience: The evidence provided for a CESR application in oncology must cover the knowledge, skills and qualifications to demonstrate the required competencies in all areas of the clinical oncology curriculum. If evidence is missing from any area of the curriculum, the application will fail.
Primary Evidence: To demonstrate that you can do what is required by the curriculum, you need to submit primary evidence of your clinical practice which shows how you work on a day-to-day basis: letters, reports, assessments etc. References, retrospective case summaries, and reflective notes can all be used in a CESR application, but by themselves they are not sufficient.
Audit and Governance: You are required to submit evidence of your active leadership in audit, including evidence that you have completed at least one audit cycle
Currency of Evidence: Your evaluators will be looking for evidence of current competency, generally defined as within the last five years. If you have completed training before this point, it is crucial that you provide evidence of maintaining competency across the whole area of the curriculum.
The GMC asks that only evidence that is strictly relevant is sent as it will help them to process the application quicker. The guidance on compiling your evidence will help you to decide what is relevant and what is not – you can find this on the GMC website here.
As a general guide, the GMC usually expects to see about 800 - 1000 pages of evidence, divided into four different domains, reflecting those of Good Medical Practice. The GMC recommends that you apportion the evidence provided as shown below:
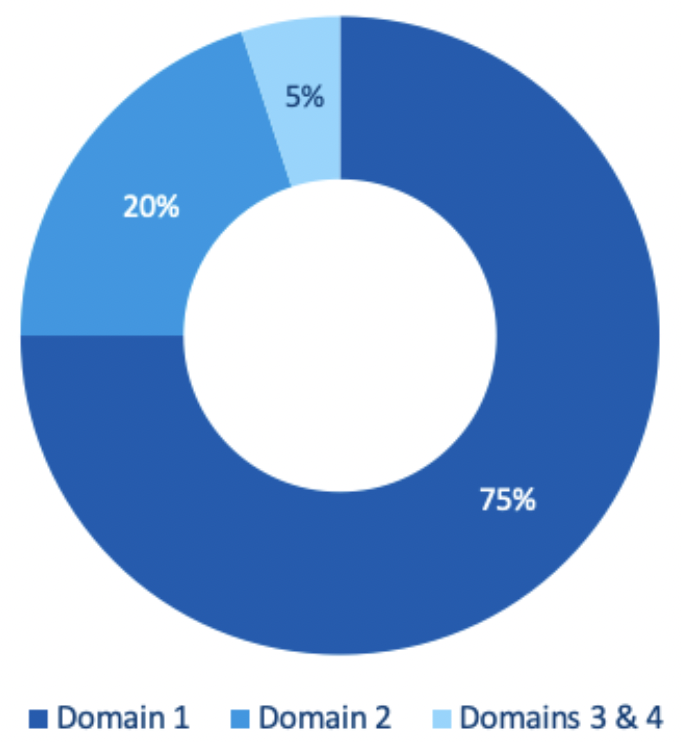
- Domain 1 – Knowledge, skills and performance
- Domain 2 – Safety and quality
- Domain 3 – Communication, partnership and teamwork
- Domain 4 – Maintaining trust
Please note, you cannot compensate for evidence lacking in one area by providing more evidence in another area.
The full list of evidence required for each domain can be found on the GMC website here.
Gathering Evidence for a CESR Application
Domain 1 – Knowledge, skills and performance
Qualifications
- Primary Medical Qualification (PMQ)
- Specialist medical qualification(s)
- Curriculum or syllabus (if undertaken outside the UK)
- Specialist registration outside the UK
- Honours and prizes
- Other relevant qualifications
Assessments and appraisals
- Appraisals and assessments
- RITAs, ARCPs and training assessments
- 360˚ and multi-source feedback
- Awards and discretionary points letters
- Personal development plans (PDP)
Logbooks, records of daily clinical practice and portfolios
- Logbooks
- Consolidation, cumulative data sheets, summary lists and annual caseload statistics
- Medical reports
- Case histories
- Referral letters discussing patient handling
- Patient lists
- Departmental (or trust) workload statistics and annual caseload statistics
- Rotas, timetables and job plans
- Courses relevant to curriculum
- Portfolios (electronic or revalidation)
Details of posts and duties (including both training and experience posts)
- Employment letters and contracts of employment
- Job descriptions
- Job plans
Research, publications and presentations
- Research papers, grants, patent designs
- Publications within specialty field
- Presentations, poster presentations
CPD and CME
- CPD record certificates, certificates of attendance, workshops and at local, national and international meetings or conferences
- CPD registration points from UK Medical Royal College (or equivalent body overseas)
- Membership of professional bodies and organisations
- Teaching timetables
- Lectures
- Feedback or evaluation forms from those taught
- Letters from colleagues
- Attendance at teaching or appraisal courses
- Participation in assessment or appraisal and appointments processes
Domain 2 – Safety and quality
Participation in audit, service improvement
- Audits undertaken by applicant
- Reflective diaries
- Service improvement and clinical governance meetings
Safety
- Health and safety
Domains 3 - Communication, partnership and teamwork
Communication
- Colleagues
- Patients
Partnership and teamwork
- Working in multidisciplinary teams
- Management and leadership experience
- Chairing meetings and leading projects
Domain 4 – Maintaining trust
Acting with honesty and integrity
- Honest and integrity
- Equality and human rights (including disability, human rights, race, religion and ethnicity awareness and equal opportunities)
- Data protection
Relationships with patients
- Testimonials and letters from colleagues
- Thank you letters, cards from colleagues and patients
- Complaints and responses to complaints
For more guidance on the different types of evidence, see the specialty specific guidance from the GMC for clinical oncology.
Validating Evidence
Original documents which are on headed paper with a hospital stamp and original signatures do not need additional validation.
All photocopied evidence should contain a hospital stamp on every page of each document, the validator’s name (printed and in full), job title (printed and in full) and original signature.
Application Submission
All CESR applications are submitted online via GMC Online and if you have not already created an account, you can find a guide on how to do so here.
Electronic evidence is required for each of the different evidence sections of the CESR application. Once started, the online application remains open for 12 months, meaning that it can be used as a portfolio to gather evidence against each of the different sections.
Your electronic evidence can be in any of the following formats:
- .doc
- .ppt
- .xls
Formats outside of these are unlikely to be accepted.
The Online Application
You will be required to complete the following sections once you begin your application:
- Specialty details
- Qualification details and professional experience
- Details of your referees
- Registration and licensing history
- Evidence summary
- Details of your verifiers
- Final declaration and payment
Additional Evidence
Once an Adviser on the Specialist Applications Team has reviewed your initial evidence, they will provide you with information on:
- What evidence they’ve accepted
- What evidence they’re unable to accept (including the reasons for this)
- Advice and guidance on how your application could be strengthened
You’ll have up to 60 days to provide additional documentary evidence in support of your application (30 days if you’ve submitted a Review application).
For further information about the online application process, see the GMC’s User Guide.
How long does it take to complete?
As there is a substantial amount of evidence to gather for a CESR application, the process of preparing all the necessary documentation and applying for CESR can take even longer than this, and a typical candidate will usually set out to complete this within 1 – 3 years.
It is worth noting that more senior oncologists, such as consultants, are more likely to have achieved all the competences outlined in the curriculum.
The indicative period of training for a CCT in clinical oncology is seven years (not including Foundation Training), so it is highly unlikely that you would achieve the competencies required for a CCT in a shorter period of time. Therefore, CESR is not suitable for more junior clinical or radiation oncologists.
Cost of CESR Applications
All oncologists applying for Specialist Registration must pay a fee. For CESR, this fee is £1,727.
For CESR-CP and CCT, the cost is £452.
How long does it take to receive a decision?
The GMC estimate that it can take between six and eight months to receive a decision, from the date you submit your CESR application.
Clinical Oncology Jobs in the NHS without CESR
Overseas doctors do not require CESR to work in the UK.
There are also roles for more senior oncologists such as a specialty doctor (SAS), specialist grade or acting consultant, where you will likely receive better pay and responsibilities that are more appropriate to your level of experience. While working in these positions, you can also collect evidence of your competences, particularly those specific to the UK clinical oncology curriculum.
Across the UK there are several NHS Trusts with well-established CESR programmes of support for oncology who have taken up a fixed term post with the view to completing CESR.
These positions also facilitate a faster route to working in UK and attaining Specialist Registration when compared to making an application for CESR from overseas, which can take an additional amount of time, depending on the country in which you completed your training.
#IMG Tips
- Research/think about the types of evidence you will need and begin to gather your evidence well in advance of making your application.
- Gather evidence prospectively – this is much easier than retrospectively trying to pull together the evidence under additional pressures.
- Make sure that your evidence is of the highest possible quality and is current – you will be assessed against the most recent curriculum.
- Ensure that the evidence you collect demonstrates your competence across the whole of the Clinical Oncology curriculum, not just your sub-specialty.
- Remember to refer to the most up-to-date Clinical Oncology CCT Curriculum and Specialty Specific Guidance for the evidence requirements in your specialty.
- Create a CESR ‘to-do list’ with sections under the GMC’s 4 domain headings – organise your evidence directly into these sections to manage your progress.
- Do not submit original documents – all your copies, other than qualifications you’re getting authenticated must be accompanied by a proformas signed by the person who is attesting to the validity and accuracy of your evidence (your verifier).
- Ask an IMG Connect recruitment specialist about NHS oncology posts with CESR support. These are not always advertised by a Trust, but we can help you find a role which aligns well with your career goals in the NHS.
- Join the online community - join the IMG Oncologists Facebook group for access to a community of like-minded clinical and radiation oncology CESR aspirants and dedicated oncology recruiters.
In this group you will find tailored resources for oncology, including guidance on CESR applications, completely free to all doctors.
You can access our IMG Oncologists community here.
Sources
https://www.jrcptb.org.uk/certificate-eligibility-specialist-registration
https://www.gmc-uk.org/-/media/documents/sat---ssg---clinical-oncology---dc3556_pdf-48456770.pdf
https://www.rcr.ac.uk/sites/default/files/clinical_oncology_curriculum_2021.pdf
Getting started
Many senior IMG oncologists looking to develop their careers through Specialist Registration with the GMC may be eligible via the CESR route, and attaining CESR can be a long but very rewarding process. Look at our introduction to CESR for clinical oncologists for a full overview.
If you have any further questions about Specialist Registration, your route to the UK, or would like guidance in finding NHS posts which offer CESR support, please get in touch with us here.
Follow us on social media through the links below for regular news and updates on the Royal Colleges, relocating to the UK and working in the NHS.
Relevant Jobs
An NHS teaching hospital in Kent is looking for a Consultant in Clinical Oncology with an interest in urology. The Oncology department comprises a team of over 40 Clinical and Medical Oncology consultants and is supported by a large team of middle-grade doctors, fellows, and trainees.
Anyone requiring portfolio support (formally known as CESR) can have their required support discussed on a case-by-case basis.
JOB REQUIREMENTS AND DETAILS
-
This Clinical Oncology job is open to both international applications and UK-based doctors, though those with European Specialist qualifications or FRCR are most encouraged to apply.CCT in Clinical Oncology or equivalent experience, including CESR pathway candidates.
-
The salary will be between £105,504 and £139,882 dependent on experience or grade.
CORE DUTIES OF THIS ROLE
-
Provide high-quality specialist care to melanoma patients attending the Kent Oncology Centre.
-
Attend relevant MDT meetings and coordinate treatment planning with other specialists.
-
Participate in departmental research initiatives and contribute to national and local clinical trials.
-
Contribute to training and supervision of junior doctors and participate in clinical governance activities.
Life in Kent
The area offers the best of both worlds: urban living in towns like Maidstone and Tunbridge Wells, and tranquil countryside villages such as Lenham or Goudhurst.
Kent boasts a wide range of excellent primary and secondary schools, including several grammar schools, and is well connected to London via high-speed rail and road. Residents enjoy easy access to historic castles, the coast, national parks, shopping, and outdoor activities—perfect for both adults and families.
Known as the “Garden of England,” Kent holds historical significance with its Roman heritage, medieval cathedrals, and coastal defense legacy, offering a unique blend of culture and natural beauty.
HOW WILL IMG CONNECT SUPPORT YOU?
When applying with IMG Connect, you’ll have the full support of an expert recruitment team who will be your recruitment and relocation partners throughout the process. We\'ll support you with:
-
CV Preparation with a bespoke session with one of our specialists
-
Application support with expert knowledge of NHS specialisms & recruitment practice
-
At least two video calling interview preparation sessions
-
Contract and offer negotiations for salary, relocation packages, tenure, and more
Once you have accepted your new role, you’ll then be supported and led throughout by one of our dedicated relocation executives who will guide you all the way to starting your new role, including:
-
Document gathering and checking
-
COS and Visa Application support, if applicable
-
Support sourcing short- and long-term accommodation
-
Travel arrangements
-
Family support for finding schools and any other aspects of pastoral care
Anyone requiring portfolio support (formally known as CESR) can have their required support discussed on a case by case basis.
JOB REQUIREMENTS AND DETAILS
This oncology job is open to both international applications and UK-based doctors, though those with European Specialist registration or FRCR are most encouraged to apply.
Anyone with an interest in gynae and upper GI oncology should apply.
The salary will be between £105,504 and £139,882 dependent on experience or grade.
CORE DUTIES OF THIS ROLE
Provide systemic therapy services for patients across UHMB sites.
Participate in multidisciplinary team meetings and support associated administrative duties.
Lead and contribute to acute oncology services across the Trust (no on-call required).
Support teaching, supervision, and mentorship for junior medical staff and trainees.
LIFE IN LANCASHIRE AND SOUTH CUMBRIA
The area is rich with excellent schools, convenient rail and road links, and beautiful scenery for outdoor enthusiasts. Historically, the region has played a key role in British shipbuilding and industrial development and today combines coastal charm with cultural heritage and modern living.
HOW WILL IMG CONNECT SUPPORT YOU?
When applying with IMG Connect you’ll have the full support of an expert recruitment team who will be your recruitment and relocation partners throughout the process. We\'ll support you with:
CV Preparation with a bespoke session with one of our specialists
Application support with expert knowledge of NHS specialisms & recruitment practice
At least two video calling interview preparation sessions
Contract and offer negotiations for salary, relocation packages, tenure and more
Once you have accepted your new role, you’ll then be supported and led throughout by one of our dedicated relocation executives who will guide you through all the way to starting to your new role including:
Document gathering and checking
COS and Visa Application support if applicable
Support sourcing short- and long-term accommodation
Travel Arrangements
Family support for finding schools and any other aspects of pastoral care
An NHS teaching hospital, in Kent is looking for a Consultant in Medical Oncology with an interest in Melanoma. The Oncology department comprises a team of over 40 Clinical and Medical Oncology consultants and is supported by a large team of middle-grade doctors, fellows, and trainees.
Anyone requiring portfolio support (formally known as CESR) can have their required support discussed on a case-by-case basis.
JOB REQUIREMENTS AND DETAILS
-
This Medical Oncology job is open to both international applications and UK-based doctors, though those with European Specialist qualifications or MRCP are most encouraged to apply.CCT in Medical Oncology or equivalent experience, including CESR pathway candidates.
-
The salary will be between £105,504 and £139,882 dependent on experience or grade.
CORE DUTIES OF THIS ROLE
-
Provide high-quality specialist care to melanoma patients attending the Kent Oncology Centre.
-
Attend relevant MDT meetings and coordinate treatment planning with other specialists.
-
Participate in departmental research initiatives and contribute to national and local clinical trials.
-
Contribute to training and supervision of junior doctors and participate in clinical governance activities.
Life in Kent
The area offers the best of both worlds: urban living in towns like Maidstone and Tunbridge Wells, and tranquil countryside villages such as Lenham or Goudhurst.
Kent boasts a wide range of excellent primary and secondary schools, including several grammar schools, and is well connected to London via high-speed rail and road. Residents enjoy easy access to historic castles, the coast, national parks, shopping, and outdoor activities—perfect for both adults and families.
Known as the “Garden of England,” Kent holds historical significance with its Roman heritage, medieval cathedrals, and coastal defense legacy, offering a unique blend of culture and natural beauty.
HOW WILL IMG CONNECT SUPPORT YOU?
When applying with IMG Connect, you’ll have the full support of an expert recruitment team who will be your recruitment and relocation partners throughout the process. We\'ll support you with:
-
CV Preparation with a bespoke session with one of our specialists
-
Application support with expert knowledge of NHS specialisms & recruitment practice
-
At least two video calling interview preparation sessions
-
Contract and offer negotiations for salary, relocation packages, tenure, and more
Once you have accepted your new role, you’ll then be supported and led throughout by one of our dedicated relocation executives who will guide you all the way to starting your new role, including:
-
Document gathering and checking
-
COS and Visa Application support, if applicable
-
Support sourcing short- and long-term accommodation
-
Travel arrangements
-
Family support for finding schools and any other aspects of pastoral care
An NHS specialist cancer centre in Lancashire and South Cumbria is looking for a Consultant in Medical Oncology with an interest in upper and lower GI cancers. The oncology department comprises a team of 8 consultants and is supported by a large team of middle grade doctors.
Anyone requiring portfolio support (formally known as CESR) can have their required support discussed on a case by case basis.
JOB REQUIREMENTS AND DETAILS
This oncology job is open to both international applications and UK-based doctors, though those with European Specialist registration or MRCP are most encouraged to apply.
Anyone with an interest in upper and lower GI oncology should apply.
The salary will be between £105,504 and £139,882 dependent on experience or grade.
CORE DUTIES OF THIS ROLE
-
Provide systemic therapy services for patients with upper and lower GI cancers across UHMB sites.
-
Participate in multidisciplinary team meetings for lung and upper GI cancer and support associated administrative duties.
-
Lead and contribute to acute oncology services across the Trust (no on-call required).
-
Support teaching, supervision, and mentorship for junior medical staff and trainees.
LIFE IN LANCASHIRE AND SOUTH CUMBRIA
The area is rich with excellent schools, convenient rail and road links, and beautiful scenery for outdoor enthusiasts. Historically, the region has played a key role in British shipbuilding and industrial development and today combines coastal charm with cultural heritage and modern living.
HOW WILL IMG CONNECT SUPPORT YOU?
When applying with IMG Connect you’ll have the full support of an expert recruitment team who will be your recruitment and relocation partners throughout the process. We\'ll support you with:
-
CV Preparation with a bespoke session with one of our specialists
-
Application support with expert knowledge of NHS specialisms & recruitment practice
-
At least two video calling interview preparation sessions
-
Contract and offer negotiations for salary, relocation packages, tenure and more
Once you have accepted your new role, you’ll then be supported and led throughout by one of our dedicated relocation executives who will guide you through all the way to starting to your new role including:
-
Document gathering and checking
-
COS and Visa Application support if applicable
-
Support sourcing short- and long-term accommodation
-
Travel Arrangements
-
Family support for finding schools and any other aspects of pastoral care
Anyone requiring portfolio support (formally known as CESR) can have their required support discussed on a case-by-case basis.
JOB REQUIREMENTS AND DETAILS
This Oncology job is open to both international applications and UK-based doctors, though those with European Specialist or FRCR (UK) qualifications are most encouraged to apply.
Anyone with an interest in breast cancer should apply.
The salary will be between £106,000 to £154,760dependent on experience or grade.
CORE DUTIES OF THIS ROLE
-Contribute to outpatient and inpatient clinical care, including the Acute Oncology Service at Velindre Cancer Centre and providing advice to Health Board colleagues when on call.
-Participate in the Assessment Unit services
-Latest news & breaking headlines
-Engage in multidisciplinary team decision-making processes.
-Opportunities to deliver services at the new Velindre Cancer Centre (VCC) in Cardiff, scheduled to open in Spring 2027
LIFE IN CARDIFF
The hospital is based in Cardiff, the capital city of Wales, offering a vibrant urban lifestyle. For those seeking a more rural living environment, the surrounding areas such as the Vale of Glamorgan provide picturesque countryside settings.
Cardiff boasts a range of educational institutions, excellent transport links including a comprehensive bus and train network, and leisure activities suitable for both adults and families. The city offers numerous parks, cultural venues, and sporting facilities.
Historically, Cardiff played a significant role during the Industrial Revolution, with its coal exports powering much of the world\'s industries. The city\'s docks were once among the busiest in the world, underscoring its importance in British history
HOW WILL IMG CONNECT SUPPORT YOU
-When applying with IMG Connect you’ll have the full support of an expert recruitment team who will be your recruitment and relocation partners throughout the process. We\'ll support you with:
-CV Preparation with a bespoke session with one of our specialists
-Application support with expert knowledge of NHS specialisms & recruitment practice
-At least two video calling interview preparation sessions
-Contract and offer negotiations for salary, relocation packages, tenure and more
-Once you have accepted your new role, you’ll then be supported and led throughout by one of our dedicated relocation executives who will guide you through all the way to starting your new role including:
-Document gathering and checking
-COS and Visa Application support if applicable
-Support sourcing short- and long-term accommodation
-Travel Arrangements
-Family support for finding schools and any other aspects of pastoral care